 中文
中文



产品详情
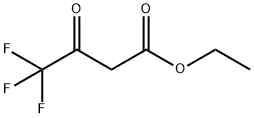
产品名称 英文名称:Ethyl 4,4,4-trifluoroacetoacetate 同义词 Ethyl 4,4,4-trifluoroacetoacetate、372-31-6、Ethyl 4,4,4-trifluoro-3-oxobutanoate、Ethyl trifluoroacetoacetate、4,4,4-trifluoro-3-oxobutanoic acid ethyl ester、Ethyl (trifluoroacetyl)acetate、Butanoic acid, 4,4,4-trifluoro-3-oxo-, ethyl ester、ETFAA、4,4,4-Triflu、4,4,4-三氟乙酰乙酸乙酯、三氟乙酰乙酸乙酯、ETFAA 产品性质 CAS编号:372-31-6 分子式:C6H7F3O3 分子量:184.11 Beilstein号:608353 EC号:206-750-7 MDL号:MFCD00000424 PubChem编号:67793 别名:4,4,4-三氟乙酰乙酸乙酯|三氟乙酰乙酸乙酯|ETFAA 英文别名:Ethyl 4,4,4-trifluoroacetoacetate|372-31-6|Ethyl 4,4,4-trifluoro-3-oxobutanoate|Ethyl trifluoroacetoacetate|4,4,4-trifluoro-3-oxobutanoic acid ethyl ester|Ethyl (trifluoroacetyl)acetate|Butanoic acid, 4,4,4-trifluoro-3-oxo-, ethyl ester|ETFAA|4,4,4-Triflu 规格或纯度:98% 英文名称:Ethyl 4,4,4-trifluoroacetoacetate 应用:合成含氟杂环医药和农药的重要中间体,有机合成砌块。 Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a general reagent to synthesize enantiopure trifluoromethyl-functionalized products. Applications include · Synthesis of (S)- and (R)-α-trifluoromethyl-aspartic acid and α- trifluoromethyl-serine from chiral CF3-oxazolidines, which is derived from ETFAA. · Enantiopure synthesis of trifluoromethyl-β-amino acid derivatives. · Synthesis of (2R)-2-trifluoromethyl-2-carboxyazetidine, (R)- and (S)-trifluoromethylhomoserines from oxazolidine intermediate obtained by condensing (R)-phenylglycinol with ETFAA. 储存温度:充氩 运输条件:常规运输 产品介绍:溶于乙醇、醚、苯等溶剂。水中溶解度10 g/l (20°C)。合成含氟杂环医药和农药的重要中间体,有机合成砌块。Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a general reagent to synthesize enantiopure trifluoromethyl-functionalized products. Applications include• Synthesis of (S)- and (R)-α-trifluoromethyl-aspartic acid and α- trifluoromethyl-serine from chiral CF3-oxazolidines, which is derived from ETFAA.• Enantiopure synthesis of trifluoromethyl-β-amino acid derivatives.• Synthesis of (2R)-2-trifluoromethyl-2-carboxyazetidine, (R)- and (S)-trifluoromethylhomoserines from oxazolidine intermediate obtained by condensing (R)-phenylglycinol with ETFAA.Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a general reagent to synthesize enantiopure trifluoromethyl-functionalized products. Applications include• Synthesis of (S)- and (R)-α-trifluoromethyl-aspartic acid and α- trifluoromethyl-serine from chiral CF3-oxazolidines, which is derived from ETFAA.• Enantiopure synthesis of trifluoromethyl-β-amino acid derivatives.• Synthesis of (2R)-2-trifluoromethyl-2-carboxyazetidine, (R)- and (S)-trifluoromethylhomoserines from oxazolidine intermediate obtained by condensing (R)-phenylglycinol with ETFAA. PubChem SID:488184012 IUPAC Name:ethyl 4,4,4-trifluoro-3-oxobutanoate INCHI:InChI=1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3 InChi Key:OCJKUQIPRNZDTK-UHFFFAOYSA-N Canonical SMILES:CCOC(=O)CC(=O)C(F)(F)F Isomeric SMILES:CCOC(=O)CC(=O)C(F)(F)F WGK Germany:2 PubChem CID:67793 UN Number:3272 Reaxy-Rn:608353 溶解性:Miscible with water, ethanol, benzene and organic solvents. 密度:1.259 折光率:1.375-1.378 闪点(℉):100.4 °F 闪点(℃):38℃ 沸点:129-130°C 熔点:-39°C 象形图: 信号词:Warning 危险声明:H315 Causes skin irritationH412 Harmful to aquatic life with long lasting effectsH302 Harmful if swallowedH226 Flammable liquid and vapor 预防措施声明:P273,P280,P370+P378,P210,P302+P352,P321,P501,P233,P240,P264,P403+P235,P303+P361+P353,P241,P270,P362+P364,P330,P242,P243,P301+P317,P332+P317 个人防护装备:Eyeshields,Faceshields,full-face respirator (US),Gloves,multi-purpose combination respirator cartridge (US),type ABEK (EN14387) respirator filter 产品包装
免责声明:以上所展示的信息由企业自行提供,内容的真实性
、准确性和合法性由发布企业负责,chemdig对此不承担任何保证责任。 同时我们郑重提醒各位买/卖家,
交易前 请详细核实对方身份,切勿随意打款或发货,谨防上当受骗。如发现虚假信息,请向chemdig举报。 |
上海阿拉丁生化科技股份有限公司
|
上海阿拉丁生化科技股份有限公司
| 邮箱 | market@aladdin-e.com |
| 联系人 | 18521732826 |
咨询请告知是在ChemDig上看到的,有助于交易达成。



